

They were discovered by James Chadwick in the year 1932 and are denoted by the symbol n or n 0.

Neutrons, similar to protons, are made of quarks and gluons. They are also found within the nucleus along with the protons in a tightly packed manner. Protons consist of even smaller particles called quarks and gluons.įound tightly packed with the nucleus, they make up virtually all of the mass of an atom, along with the neutrons.
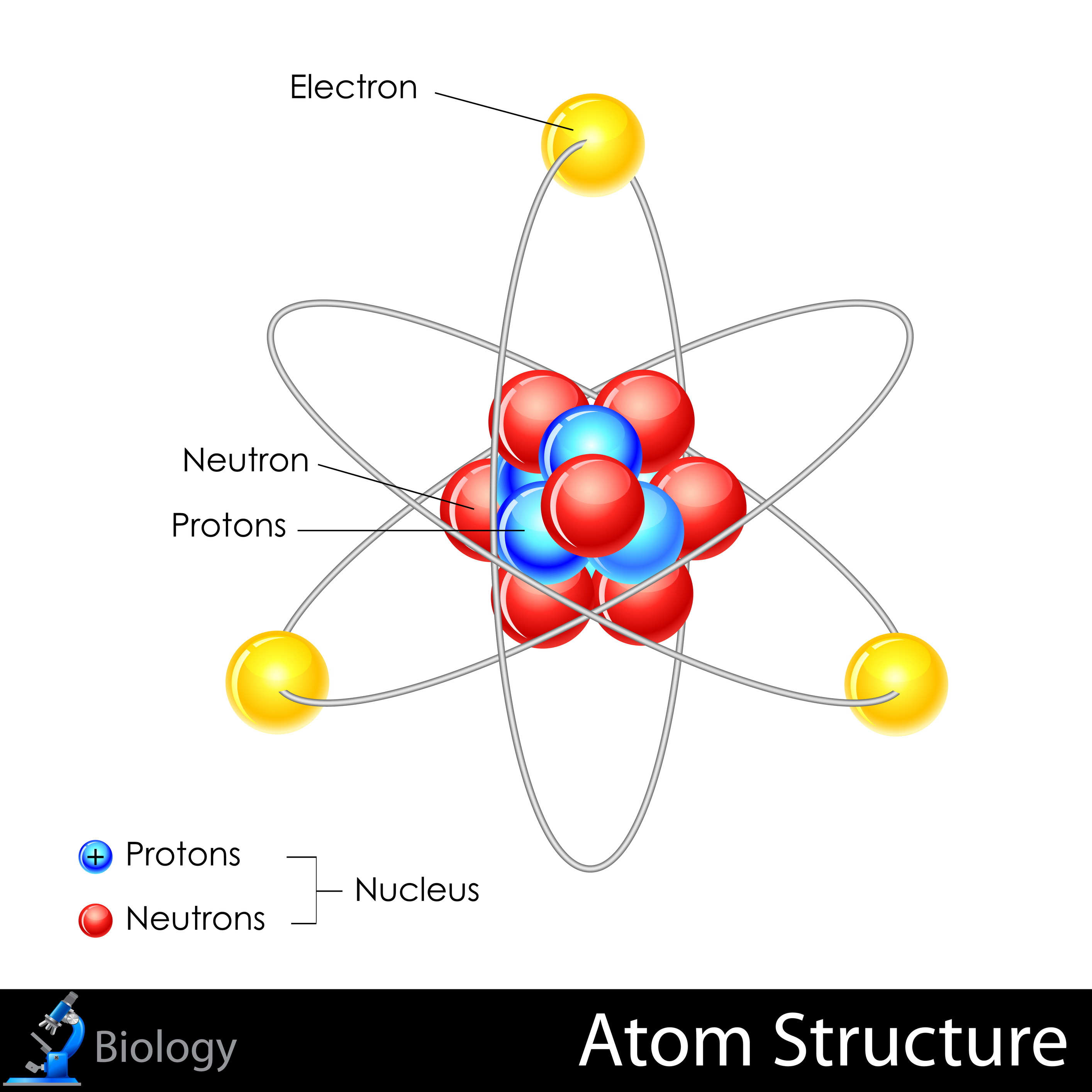
They were discovered by Ernest Rutherford in the year 1917 and are denoted by the symbol p or p +. Protons are positively charged particles found within a dense region at the center of the atom called the nucleus. When the number of negatively charged electrons is equal to the number of positively charged protons, the atom is neutral in charge. Thomson discovered it in the year 1897.Įlectrons move so fast around the nucleus that their exact location within an atom cannot be determined with accuracy. The standard symbol used for an electron is e or e –. Unlike protons and neutrons, electrons are fundamental particles much smaller (almost 1800 times) in size than protons and neutrons. They are negatively charged particles that revolve around the nucleus in a fixed orbit. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. All atoms except hydrogen contain three basic subatomic particles: 1) electrons, 2) protons, and neutrons.


 0 kommentar(er)
0 kommentar(er)
